Amino Acids | |
| Updated 2021-03-28 | |
| Amino Acids - the Building Blocks of Proteins |
![]()
One of the many different nitrogenous substances in foods is protein. Proteins are build of linear chains of amino acids. If you let the letter
R represent the different amino acids in occurring groups, all α-amino
acids, which make up the major part of the proteins, can be symbolized as
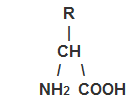
The indication, α-amino acid, means that the amino acids has one
amino group (-NH2) bound to the C-atom, which is closest to
the carboxylic group (-COOH). When the amino acids are combined into
proteins, one amino acid's -COOH group connects to another amino acid's
-NH2 group forming a socalled peptide bond while releasing
water
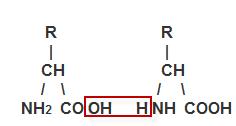
The α-amino acids are the most commonly occurring amino acids in nature. However, also other amino acids exist named β-, γ-, δ-, etc. amino acids depending on the position carbon atom the other amino acid molecule is bound to. For more information on amino acid structures, see the IUPAC-IUB Recommendations 1983 [1].
In calculations of protein from the sum of amino acid residues, it is important to make adjustments for the water involved in the condensation reaction, when the peptide bond is formed. Se more about this below.
| Amino Acids in Nutrition |
![]()
In food proteins, 20 α-amino acids occur. The amino acids are divided into groups accoding to their properties.
Traditionally, the amino acids are divided into two groups according to their importance in nutrition
- the essential (or indispensable) amino acids, which the human
body cannot synthesize itself; they comprise lysine,
methionine, threonine, valine, isoleucine, leucine, phenylalanine,
arginine, and histidine;
- the non-essential (or dispensable), because the human body
cannot synthesize them; they comprise alanine, arginine, asparagine,
aspartic acid, cysteine, glutamic acid, glutamine, glycine,
proline, serine, and tyrosine;
However, this division of the amino acids is not completely clear, because under certain conditions some of the non-essential amino acids may be considered conditionally essential.
Other groups comprise according to the characteristics of the side chains
- the sulphur-containing amino acids: methionine and cysteine;
- the aromatic amino acids: phenylalanine, tryptophan, tyrosin;
- aliphatic amino acids: alanine, glycine, isoleucine, leucine,
proline, valine;
- acidic amino acids: aspartic acid, glutamic acid;
- amidic amino acids: asparagine, glutamine;
- basic amino acids: arginine, histidine, lysine;
- hydroxylic amino acids: serine, threonine;
- etc.
The amino acids forming proteins are always the L- form of the amino
acids. According to [1],
the prefix may be omitted where the amino acid is stated to be or is
obviously derived from a protein source and is therefore assumed to be
L.
In the following, the amino acid names are therefore listed without
designation as it it always understood to be the L- form.
| Amino Acids - What is Analysed? |
![]()
The details of the analytical determination of amino acids are
described in
Greenfield
and Southgate, pp. 105-106. A
very important step in the sample
preparation before determination of the amino acid content in the sample
is the hydrolysis of the proteins. During the hydrolysis
the proteins are broken down into peptides and further to the single amino acids.
Loss and degradation of amino acids during hydrolysis is most likely,
and it is one of the principal sources of error in the determination of
amino acids. Especially, tryptophan and cysteine are very sensitive to
acids and may be destructed completely unless specific precautions are
taken.
In the following table the trivial names of the 20 α-amino acids are
listed with their symbols and codes (see also [1])
together with their ChEBI [2] Identifier, average molecular mass and
conversion factor from amino acids to amino acid residues:
| Amino acid | Symbol | Code | ChEBI Id | Average mass | Conversion factor | |
| Alanine | ala | A | 16977 | 89.09322 | 0.798 | |
| Arginine | arg | R | 16467 | 174.20112 | 0.897 | |
| Asparagine* | asn | N | 17196 | 132.11800 | 0.864 | |
| Aspartic acid* | asp | D | 17053 | 133.10272 | 0.865 | |
| Cysteine | cys | C | 17561 | 121.15922 | 0.851 | |
| Glutamine* | gln | Q | 18050 | 146.14458 | 0.877 | |
| Glutamic acid* | glu | E | 16015 | 147.12930 | 0.878 | |
| Glycine | gly | G | 15428 | 75.06664 | 0.760 | |
| Histidine | his | H | 27570 | 155.15468 | 0.884 | |
| Isoleucine | ile | I | 17191 | 131.17296 | 0.863 | |
| Leucine | leu | L | 15603 | 131.17296 | 0.863 | |
| Lysine | lys | K | 18019 | 146.18764 | 0.877 | |
| Methionine | met | M | 16643 | 149.21238 | 0.879 | |
| Phenylalanine | phe | F | 17295 | 165.18918 | 0.891 | |
| Proline | pro | P | 17203 | 115.13050 | 0.844 | |
| Serine | ser | S | 17115 | 105.09262 | 0.829 | |
| Threonine | thr | T | 16857 | 119.11920 | 0.849 | |
| Tryptophan | try | W | 16828 | 204.22526 | 0.912 | |
| Tyrosine | tyr | Y | 17895 | 181.18858 | 0.901 | |
| Valine | val | V | 16414 | 117.14638 | 0.846 |
*Sometimes it is not possible two differentiate two closely related amino acids, therefore we have the special cases
- Asparagine/Aspartic acid (asx, B)
- Glutamine/Glutamic acid (glx, X)
and data for the total of the components in these two groups will usually be expressed as aspartic acid and glutamic acid, respectively.
Similarly, one will often find data on cystine (ChEBI:16283, avg. mass 240.30256) instead of cysteine.
Cystine is a common side-chain modification of cysteine yielded by
oxidation of two molecules of cysteine and is generally regarded as
the more stable than cysteine. The oxidation is usually carried out in
order to protect cysteine (and methionine) before hydrolysis.
The average molecular mass of cystine, 240.30256, is very close to twice the average molecular mass of cysteine. Therefore, no
specific provisions (recalculations) are needed for data expressed as cystine
as compared to cysteine.
This leaves the 18 amino acids that are normally reported: alanine, arginine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine.
In addition, several amino acid derivatives, such as hydroxylysine and hydroxyproline, are analysed in for example meat and meat products. Both components are present in high amounts in connective tissue (collagen), and the result of the determination of the hydroxy amino acids is used as a quality measure. A high content of hydroxylysine or hydroxyproline is a sign of the use of low quality meat (high amounts of connective tissue).
| Amino Acids - Expression and Presentation |
![]()
When analysing for amino acids, traditionally also total nitrogen is
analysed in the samples, and the amino acid profile, i.e. the amounts of
all corresponding amino acids in a food, is normally expressed as g or
mg (gram or milligram) amino acids per g nitrogen (or 16 g nitrogen).
The use of the nitrogen content of each sample as the basis for
expressing amino acid content eliminates variations among samples of a
food due to different levels of other constituents, e.g. different
moisture or fat content.
In addition, amino acids are usually expressed as g or mg per 100 g edible food. However, the amino acid profiles are the basis for this expression, and in most cases amino acid content is calculated from the amino acid profile (g og mg per g N) to the actual content in the food (g or mg per 100 g edible portion of food).
According to Greenfield and Southgate, Table 9.1, the preferred unit for amino acid values is mg. In many cases, it may however be more convenient to use the unit g - especially with regard to the number of significant digits.
The maximum number of significant digits is similarly for amino acids given as 3. Due to the extensive losses and degradations mentioned above, this number of significant digits may be somewhat too high for older amino acid data. This is also the case, if data are recalculated. It is therefore suggested that for older amino acid data only 2 significant digits may be used.
| Amino Acids - Calculation of Protein Content |
![]()
In the
FAO Food and Nutrition Paper 77 [3], the preferred method for
measuring protein is stated as
"the sum of individual amino acid
residues (the molecular weight of each amino acid less the molecular
weight of water)".
This is the most precise method of calculating
the protein content of foods; however, the amino acid data currently
available do
not cover all the foods needed in a comprehensive food composition
database.
The sum of the amino acid residues is a more precise measure for protein than the calculated protein content based on Kjeldahl, Dumas, or other anlytical methods for the determination of total nitrogen.
The factor needed to calculate the amino acid residue for each amino acid before the summation is given in the table above, i.e. first calculate the amino acid residue for each amino acid by multiplying the amino acid value with its corresponding conversion factor, then make the summation of all amino acid residue values.
| Amino Acids - Sources of data |
![]()
There are numerous sources of data for amino acids, but is
characteristic for amino acid data that it is mostly raw foods that has
been analysed. A simple search for amino acids in the bibliographic
databases will reveal an enormous amount of references.
In addition, there are a collection of composite works, tables of amino
acids, from the last half century worth mentioning:
Harvey's Tables of the amino acids in foods and feedingstuffs [4] forms together with Kuppuswamy et al's Proteins in foods [5] and Orr and Watt's Amino Acid Content of Foods [6] the comprehensive basis for the compiled works on amino acids in foods published after 1960.
The Amino Acid Content of Foods by M. L. Orr and B. K. Watt from
1957 [6] gives a summary of data available for 18 amino acids in terms
of amino acid content per gram of nitrogen present in the food. For each
of the 202 foods, the average (mean), the maximun, and the minimum
values are shown. In addition, the number of values (data points)
selected for use in obtaining the mean is also shown.
The second table lists the average amino acid contents of foods per 100
grams of edible portions. These values are calculated from the average
amino acid content given in the previous table.
In Denmark, Bjørn O. Eggum was very active in amino acid analysis from the 1960's up into the 1990's and published a long range of scientific papers on amino acids and protein quality in foods and feeds, and he was coauthor of even more. Bjørn Eggum published in 1968 Aminosyrekoncentration og proteinkvalitet [7], a table with amino acid data for 17 amino acids as well as protein quality data in 90 foods and feeds. Data are given in g per 16 g nitrogen. The amino acids values were determined by chemical method with an amino acid analyzer.
FAO's Amino-Acid Content of Foods and biological data on proteins [8] published in 1970 contains data compiled from scientific literature 18 amino acids and protein quality data for 339 foods. The tables list the values for the moisture, nitrogen, and protein content in g per 100 g edible portion as well as the nitrogen-to-protein conversion factor used. Average amino acid values are listed in mg per g nitrogen. The tables distinguish between chemical methods (and more precisely column chromatography) and microbiological methods.
In McCance and Widdowson's The Composition of Foods, 4th ed. [9] Paul and Southgate published in Section 2 a table of 18 amino acids (mg per g nitrogen) for 126 foods. The main sources of data are FAO's table [8] mentioned above with new analytical data on milk, meat and miscellaneous products. In the First Supplement [10] to this publication, the amino acid content has been calculated (mg per 100 g edible portion) for most of the foods in [9].
Helge Søndergaard published in 1984 Aminosyreindholdet i danske levnedsmidler (the Amino Acid content of Danish Foods) [11] with data for 18 amino acids (mg per 100 g edible food) in 188 Danish foods. The amino acid analysis was carried out with microbiological methods.
The dissertation of Pirjo Salo-Väänänen [12] from 1996 contains data for 18 amino acids (mg per 100 g edible food) in 148 Finnish foods. This work is also interesting as it questions the established nitrogen-to-protein conversion factors and concludes that the Jones' factors used are too high, the net protein value (defined as sum of amino acid residues) of foods is about 20% lower than the protein value calculated from crude protein (Kjeldahl, Dumas, etc.).
Since then most of the work has been on a few foods/species at a time. However, worth mentioning is the work of Kim et al [13] published in 2009. The paper contains amino acid data for 150 Korean foods.
The FAO/INFOODS datasets on pulses [14] and fish and shellfish [15] contain comprehensive information on proximates, minerals and vitamins also information on amino acid s compiled from scientific literature and other sources. The dataset for fish and shellfish also include calculations of nitrogen-to-protein conversion factors on species level based on the amino acid information from the literature.
Data from these works will be transformed to the AminAcidBase™ database on amino acid content in foods (in progress).
| References |
![]()
1. IUPAC-IUB: Nomenclature and symbolism for amino acids and peptides
(IUPAC-IUB Recommendations 1983)". Pure Appl. Chem. 56 (5): 595–624,
1984.
DOI: 10.1351/pac198456050595
-
full text pdf.
2. EMBL-EBI: Chemical Entities of Biological Interest (ChEBI). Dictionary of molecular entities focused on ‘small’ chemical compounds.
WWW:
https://www.ebi.ac.uk/chebi/init.do.
3. FAO: Food Energy - methods of analysis and conversion
factors. FAO Food and Nutrition Paper 77, FAO, Rome , 2003.
WWW:
ftp://ftp.fao.org/docrep/fao/006/y5022e/y5022e00.pdf.
4. Harvey D: Tables of the amino acids in foods and feedingstuffs. Farnham Royal, Bucks., Commonwealth Bureaux. Commonwealth Bureau of Animal Nutrition, Technical Communication No. 19, 1956.
5. Kuppuswamy S, Srinivasan M and Subrahmanyan V: Proteins in foods. New Delhi, Indian Council of Medical Research. Special Report Series No. 33, 1958.
6. Orr, ML and Watt BK: Amino Acid Content of Foods. Home
Economics Research Report No. 4. United States Department of
Agriculture, Washington, D.C., December 1957.
WWW:
http://toolbox.foodcomp.info/References/AminoAcids/USDA -
Amino Acid Content of Foods - Home Economics Research Report No. 4.,
December 1957.pdf
7. Eggum BO, Landøkonomisk Forsøgslaboratorium: Aminosyre
concentration og proteinkvalitet. Stougaards Forlag, København 1968 (in
Danish with English food names).
WWW:
http://toolbox.foodcomp.info/References/AminoAcids/Eggum -
Aminosyrekoncentration og Proteinkvalitet. Stougaards Forlag, København
1968.pdf
8. FAO Food Policy and Food Science Service, Nutrition
Division: Amino-Acid Content of Foods and biological data on proteins.
FAO Food and Nutrition Series No. 21. Food and Agriculture Organisation
of the United Nations, Rome 1970.
WWW:
http://www.fao.org/DOCREP/005/AC854T/AC854T00.htm
9. Paul AA and Southgate DAT: McCance & Widdowsons's The Composition of Foods, Fourth Revised Edition. MRC Special Report No. 297. Elsevier/North-Holland Biomedical Press, November 1978.
10. Paul AA, Southgate DAT, Russell J: First supplement to McCance & Widdowsons's The Composition of Foods (Amino acids, mg per 100 g food, Fatty acids, g per 100 g food). HMSO Her Majesty's Stationary Office, 1980.
11. Søndergaard H: Aminosyreindholdet i danske
levnedsmidler (the Amino Acid content of Danish Foods). Publikation
nr. 98. Statens Levnedsmiddelsinstitut, 1984.
WWW:
http://toolbox.foodcomp.info/References/AminoAcids/DOC00153.pdf
12. Salo-Väänänen P: Determination of protein content in foods by the amount of total nitrogen or amino acids (Diss., in Finnish). EKT series 1050. University of Helsinki. Department of Applied Chemistry and Microbiology, 1996.
13. Bok Hee Kim, Haeng Shin Lee, Young Ai Jang, Ji Yeon Lee,
Young Ju Cho, Cho-il Kim: Development of amino acid
composition database for Korean foods. Journal of Food Composition
and Analysis 22 (2009) 44–52. DOI:
10.1016/j.jfca.2008.07.005.![]()
14. Fernanda Grande, Barbara Stadlmayr, Morgane Fialon, Sergio
Dahdouh, Doris Rittenschober, T Longvah & U. Ruth Charrondiere:
FAO/INFOODS global food composition database for pulses. Version 1.0 -
uPulses1.0.
Food and Agriculture Organization of the United Nations, Rome, 2017.
- User
Guide
![]() -
Datasheet
-
Datasheet
![]()
15. Doris Rittenschober, Anders Møller, Barbara Stadlmayr,
Sarah Najera Espinosa, & U. Ruth Charrondiere:
FAO/INFOODS global food composition database for fish and shellfish,
version 1.0 - uFiSh1.0.
Good and Agriculture Organization of the United Nations, Rome, 2016.
- User
Guide
![]() -
Datasheet
-
Datasheet
![]()
![]()
| ||
| ||
| ||
| ||
|